What Word Chemical Equation Describes the Second Chemical Reaction
A chemical reaction transforms one or more substances into a set of different substances. A chemical equation shows the starting compound sthe reactantson the left and the final compound sthe productson the right separated by an arrow.
Balancing Chemical Equations Chemistry 10
Equations 411 and 412 are balanced chemical equations.

. 2 2 4. The two way arrows are used to show that. During this process atoms are rearranged but nothing new is.
As a word equation or as Hydrogen and oxygen react to form water or Water is made by reacting hydrogen and oxygen. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. A chemical equation describes the changes state chemical composition and heat energy that take place during a chemical process.
A chemical reaction is described by achemical equation an expression that gives the identities and quantities of the substances involved in a reaction. They can be used to find areas of low or high pressure over a broad area and they can tell us how intense the system may be. The reactions of matter whether occurring in natural processes or in the laboratories can be interpreted using another language of chemistry the equation.
Here is as an example the chemical equation for rusting. Describe the substances that react in a chemical reaction termed reactants and the products that are formed along with their states formula equations are a shorthand. 4 4 yes.
Hydrogen Oxygen Water with electric sparks Reactants are written at first and then an arrow à is written means forms or gives and then products are written. It is critical to balance the equation after writing the chemical reaction. What is different on each side of the equation is.
Each side of the reaction has two chromium atoms seven oxygen atoms two nitrogen atoms and eight hydrogen atoms. A Bunsen burner is commonly used to heat substances in a chemistry lab. The above reaction while technically correct does not account for the dissociation of the sodium acetate in water.
Many chemical equations also include phase labels for the substances. For example the reaction between magnesium and oxygen to give magnesium oxide can be represented as follows. Magnesium Oxygen Magnesium oxide.
Isobars are lines of constant or equal pressure on a weather map. WRITE THE WORD EQUATION AND CHEMICAL EQUATION REPRESENTING THE FOLLOWING REACTIONS. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen.
Mg s2H2O l - Mg OH2 aqH2 g Mg l2H2O g - Mg OH2 aqH2 g Click card to see definition. Explain how the two reactions are different from each other. A chemical equation is a short way to represent a chemical reaction the process in which substances are turned into other substances.
It is necessary to know the formulae of the elements or compounds involved in the chemical reaction in order to write the chemical equation. Fe O2-- Fe2O3 Equations that just show the formulas of the reactants and products are called formula. Tap card to see definition.
Another common way to write this reaction is. Magnesium hydrochloric acid - magnesium chloride hydrogen What is the source of the atoms in the hydrogen gas produced by this reaction. Word equations.
If you were to describe a chemical reaction in a sentence it would be quite long. N 2 3H 2 2NH 3. Reactants Product Substances.
The word equation for this reaction is. TextMethane textoxygen rightarrow textcarbon dioxide textwater Figure PageIndex2. The l stands for low pressure and h stands for high.
S for solid ℓ for liquid g for gas and aq for aqueous ie dissolved in water. The following word equation describes a chemical reaction in which hydrogen is produced. With s solid l liquid g gas aq aqueous or in water solution.
2HCl aq CaCO3 s CO2 g CaCl2 aq H2O l hydrochloric acid and solid calcium carbonate react to produce carbon dioxide water and aqueous calcium chloride. H2 g Cl2 g -- 2HCl g Write a sentence that completely describes the chemical reaction represented by this. The formulas of the reactants on the left are connected by an arrow with the formulas of the products on the right.
On weather maps you may have noticed areas that have a large l or h over a region with lines circling around them. Hydrogen gas oxygen gas steam. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia NH 3.
1 2 2 1 4. To express it in a shorter form we can write it as a word-equation. NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2.
The chemical reaction 2 H 2 g O 2 g 2 H 2 O g would be expressed as. The substances that enter into a chemical reaction are called reactants and the substances formed are the. Hypothesize any difference you might be able to observe in these two reactions represented by the following equations.
In a balanced chemical equation both the numbers of each type of atom and the total charge are the same on both sides. Up to 24 cash back A chemical equation is a representation of a chemical reaction. The chemical reaction expressed in terms of full names of reactants and products is called word equation.
Word Equation Examples. SOLID POTASSIUM K AND CHLORIND Cl₂2 GAS COMBINE TO FORM POTASSIUM CHLORIDE KCI POWDER.
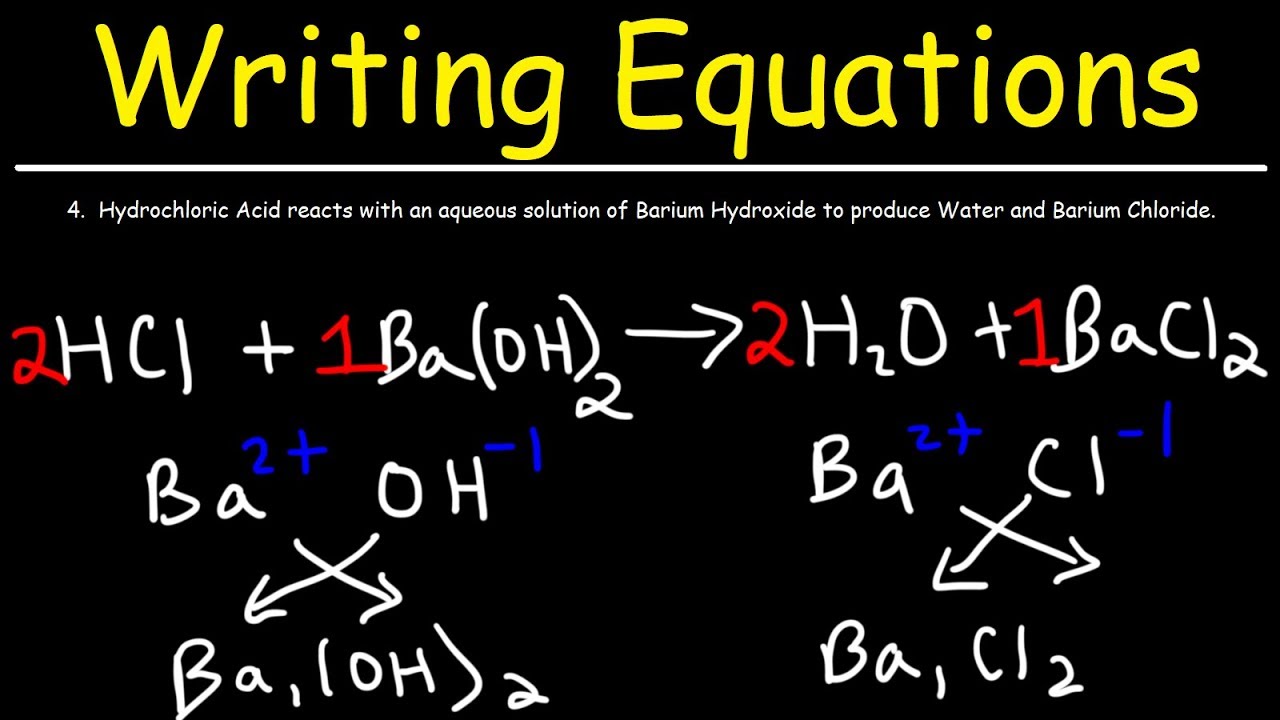
How To Write Chemical Equations From Word Descriptions Youtube
Chemical Equations Chemistry Encyclopedia Reaction Water Elements Examples Gas Number Salt Molecule

Chemical Reactions 3 Of 11 Combustion Reactions An Explanation Youtube

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
0 Response to "What Word Chemical Equation Describes the Second Chemical Reaction"
Post a Comment